Stereochemistry Lab
by Isaiah Sippel · Aug 7, 2025
Introduction
In chemistry, two molecules can have identical molecular formulæ and yet drastically different properties. This concept of isomerism, where two molecules can have the same formula but different structures, can have great effect on a molecule's chemical properties. While it is obvious that changing the bonding arrangement in a molecule (constitutional isomerism) will have a drastic effect on the molecular properties (for example, cyclohexane vs. hexene), it may be less obvious that molecules with the same bonding pattern can also have different properties. Stereoisomers have the same atoms and bonding arrangements, but the atoms are positioned in a way that make them non-superimposable (just like your left and right hands).
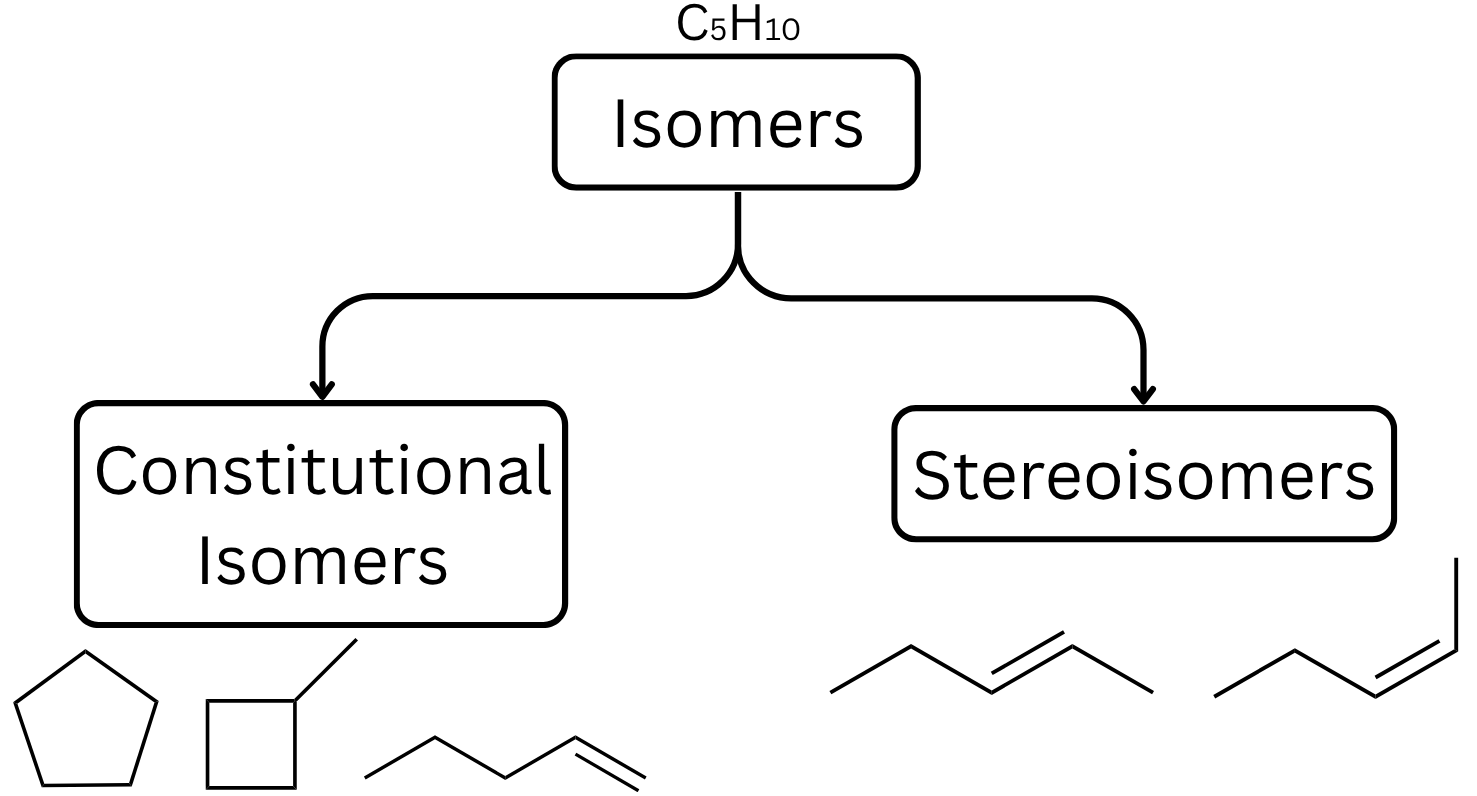
Understanding the subtle differences between stereoisomers is often difficult with simple 2D representations of the molecules (and isn't always easy even with 3D structures). This lab will use 2D and 3D representations to introduce various different kinds of stereoisomers, including cis–trans isomers, enantiomers, and diastereomers.
Cis–Trans Isomers
Cis–trans isomers are among the simplest examples of stereoisomerism. The cis and trans prefixes correspond to the arrangement of functional groups relative to a plane, with cis indicating that the groups are on the same side, and trans indicating that they are on opposing sides.
Intro to Cis–Trans Isomers
Example 1: 2-Butene
One of the most basic examples of cis–trans isomerism is 2-butene.
The double bond in the center of the molecule locks the orientation of its substituents and gives us a pair of cis–trans isomers for this molecule. Pictured above is the trans configuration of 2-butene, where the two methyl groups lie across from each other on either side of the double bond. Draw the cis configuration of 2-butene below, then optimize each structure in Rowan by following the subsequent directions.
- Log in to Rowan, enter a project, and create a new folder called "Stereochemistry Lab."
- Click on
Calculation>Draw 2Dand draw the trans configuration of 2-butene (pictured above) by selecting single bond from the menu on the left side of the viewer, then clicking and dragging to create your bonds. When you are done, clickSavein the top right. - Click
Draw 2Dagain, and this time draw the cis configuration. When complete, clickSave, then click the cube to the right of each SMILES string to convert to 3D. - Set the
Level of Theoryto "AIMNet2," selectOptimizeandFrequencies, and clickSubmit(this will submit both structures at once).
Once the calculations have completed, look at the energy of each resulting isomer (you can select both to get the relative energy and see them superimposed). Which molecule has a lower absolute energy and how much lower is it? What might cause it to be lower in energy?
Example 2: 1,2-Difluoroethylene
The 1,2-difluoroethylene molecule, F–C=C–F, has cis–trans isomerism. Draw both the cis and trans structures of 1,2-difluoroethylene below.
The cis configuration of 1,2-difluoroethylene is more stable than the trans configuration. Why do you think that might be?
Example 3: 1-Chloro-1,2-Difluoroethylene
1-Chloro-1,2-difluoroethylene has the same structure as 1,2-difluoroethylene, but with an added chlorine substituted for a hydrogen. When there are more than 2 non-hydrogen groups connected to a double bond, the cis–trans system becomes ambiguous and you need to switch to E/Z nomenclature. E/Z nomenclature uses Cahn–Ingold–Prelog priorities, where a higher atomic number for a substituent generally indicates a higher priority (there are a few other rules but only if it's a tie). Then, if the highest two priority substituents are on the same side of the double bond it's notated as (Z), and if the highest two priority substituents are on opposing sides of the double bond, it's notated as (E).
Chlorine has a higher priority than fluorine (and fluorine has a higher priority than hydrogen). Here, the compound where the chloro and the fluoro are on the same side of the double bond is (Z) and the compound where where the chloro and the fluoro are on opposite sides of the double bond is (E).
Identify which of the above is the (Z) and which is the (E) isomer. Which isomer is more stable?
Example 4: Cyclooctene
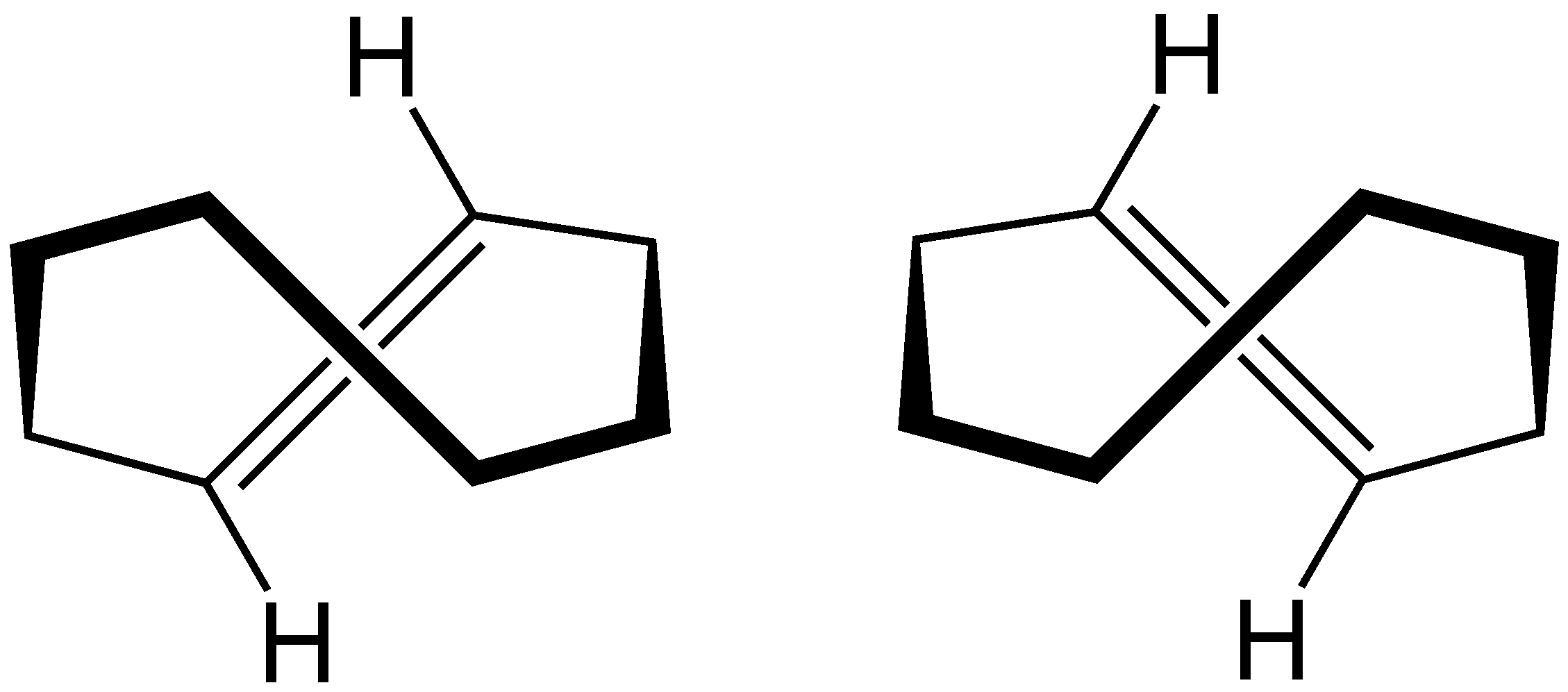
The image above shows two trans configurations of cyclooctene. Draw what the cis configuration looks like and then calculate its energy in Rowan. Do you expect it to be higher or lower energy than that of trans-cycloooctene?
Can a molecule with cis–trans isomerism switch between these configurations freely? What would be required for such a transition?
Enantiomers
Enantiomers are a type of stereoisomer that share the same chemical composition but differ in the three-dimensional arrangement of their atoms. More specifically, enantiomers are isomers that are non-superimposable mirror images of each other, meaning one cannot be converted into the other without reflection.
Thalidomide
Thalidomide is one of the most famous examples of the impacts of enantiomers. Thalidomide started being produced as a medicine in 1950s Germany to help treat morning sickness in pregnant women. What they did not know when they began selling thalidomide was that thalidomide had two enantiomers, the (R)- and (S)-enantiomers, and it is commonly taught that each has very different properties. The story goes that the (S)-enantiomer did indeed have sedative and anti-nausea properties, the (R)-enantiomer caused severe birth defects.
However, when thalidomide is in the body, it racemizes, or rapidly converts from one enantiomer to the other, ultimately making a 50/50 racemic mixture of the two enantiomers. In this way, regardless of what enantiomer is ingested, both the medicinal properties and birth-defect side effect will be present. It is also because of this racemization that we can't really be sure if either enantiomer is more beneficial or harmful than the other.
It is estimated that roughly 10,000 babies in total were born with severe birth defects caused by thalidomide, with an infant mortality rate of roughly 40% for those badly affected, likely due to internal organ damage.1
Examples of Enantiomers
Example 5: Methane with Halogens
These two methanes are enantiomers of one another. Is there any way that they can be rotated to have the same structure? Try dragging the molecules to orient them to look identical.
Without anything acting upon it, can one of these enantiomers easily convert into the other's orientation?
Example 6: Enantiomers with Multiple Stereocenters
When a molecule has multiple stereocenters (in organic chemistry this is often a carbon with 4 bonds), it can still have mirror-image enantiomers. However, for this to be the case, every stereocenter must be inverted, not just one. If not every stereocenter is inverted, that is not an enantiomer.
Can you think of any cases in which every stereocenter could invert, but no change is made?
Diastereomers
If some but not all stereocenters of a molecule are inverted, the resulting molecule is a diastereomer of the original molecule.
Example 7: Tartaric Acid
Tartaric acid is one of the simpler examples of a diastereomer. Tartaric acid has two stereocenters, and because of this there are three stereoisomers of tartaric acid, with two being enantiomers of each other, and the last being a diastereomer of the other two.
Draw the other enantiomer of tartaric acid, as well as its diastereomer. (Hint: draw in the hydrogens!)
Example 8: 3-Bromo-2-Butanol (Reference)
With a very similar structure to tartaric acid, 3-bromo-2-butanol has a four carbon structure, with constituents added to its carbons. However, 3-bromo-2-butanol has four total stereoisomers, and only has constituents on its two central carbon atoms.
Draw any diastereomer of the given structure of 3-bromo-2-butanol by inverting one of its stereocenters, then draw the enantiomers of both structures. This should result in four unique stereoisomers.
Pick any two stereoisomers of 3-bromo-2-butanol that are diastereomers (not enantiomers!) of each other, and model each with Rowan by following the directions below:
- In the folder you created earlier, click on
Calculation>Draw 2D. - Draw the first stereoisomer of your choice, using the
Single Bondtool (in the left toolbar) and by selecting your molecules on the right. To specify the stereochemistry, right-click on the bond with stereochemistry you want to specify and selectSingle UporSingle Down, depending on which is appropriate. - Click
Save, then click the cube next to the SMILES formula toConvert to 3D. - Select "AIMNet2" as your level of theory, check the boxes for
OptimizeandFrequencies, and clickSubmit Calculation.
Compare the energies of each diastereomer you calculated. What do you notice? If the energies are different, why do you think that is?
Advanced Stereochemistry
Platin
Platin, also called diammine platinum dichloride, is a molecule that, at a glance, might not look like it has cis-trans isomerism. With four atoms bonded to a central platinum, you might think that the molecule would form a tetrahedral geometry. However, because platinum has 8 electrons in its d-orbital, the most favorable way for the d-orbital to split and for the ligands to bond to platinum results in a square planar geometry.
Even though the molecule does not contain a double bond, platin still displays cis-trans isomerism. Is the isomer pictured above in the cis or trans configuration?
Platin is also a great example of a non-tetrahedral stereocenter. Metal complexes in particular are able to exhibit stereocenters for just about any molecular geometry with at least four ligands, for example tetrahedral, trigonal bipyramidal, or square planar (like platin).
Atropisomers
Atropisomers are stereoisomers that differ by only a rotation about a single bond. However, rotation about a single bond is free, so it might seem unusual that atropisomers would not rapidly interconvert. However, sometimes the rest of the molecule can physically get in the way of that free rotation through steric strain, raising the barrier for rotation to the point that we can call them atropisomers (from Greek: ατροπος, meaning "not to be turned"). Take a look at this scan of an atropisomer being rotated 360º about its single bond.
What do you notice about the potential energy graph? When is the molecule in a stable orientation?
Based on this potential energy graph, how many stable stereoisomers does this molecule have?
What might be causing the increase of potential energy as the molecule rotates?
Additional Resources
We have a tutorial that walks through the process of modeling atropisomers using dihedral scans (like the BINAP scan above).
3Blue1Brown made a fascinating video about chirality in action, showing how sugar water can rotate light and create a barber pole effect.
Footnotes
-
Vargesson, N. Thalidomide-induced teratogenesis: History and mechanisms. Birth Defects Res. C Embryo Today 2015, 105 (2), 140–156. https://doi.org/10.1002/bdrc.21096. ↩