Diels–Alder Lab
by Isaiah Sippel · Jul 30, 2025
Overview
This lab introduces the basics of the Diels–Alder reaction: what it is, how it works, and how the rate can be tailored by adding substituents.
What Is a Diels–Alder Reaction, Anyway?
The Diels–Alder reaction is a fundamental reaction in organic chemistry in which a diene (a molecule with two conjugated double bonds) reacts with a dienophile (which contains a π-bond) to form a six-membered ring.
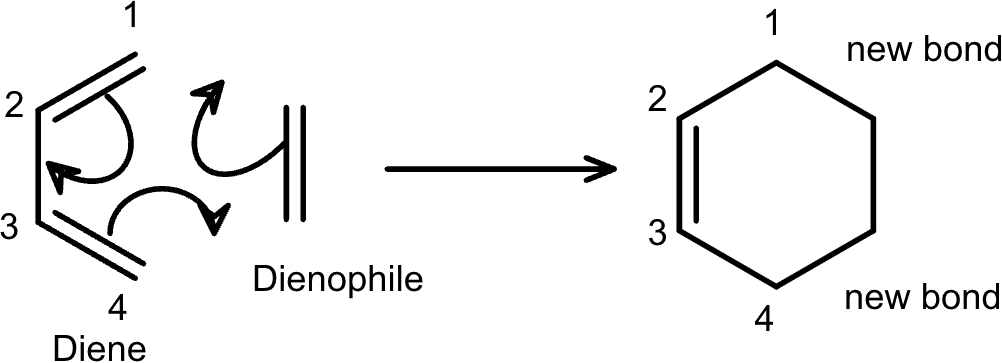
In this reaction, three π-bonds are broken, two σ-bonds are formed, and one new π-bond is created.
Let's start by examining the most basic version: C4H6 + C2H4.
This reaction involves only a diene and a dienophile. It is exothermic, as shown in the potential energy diagram below (press the play button to animate the reaction).
Predicting Activation Energy and Reaction Energy
To calculate the energies involved in this reaction, we need to know the energy of the reactants, transition state, and product.
Reactants and Products
- Log in to Rowan, and create a new folder called Diels–Alder Lab.
- Click on
Calculation>Draw 2Dand draw a butadiene molecule, C=C–C=C, by selecting single bond from the menu on the left side of the viewer, then clicking and dragging to create your bonds. Click again on a bond to make it a double-bond. Save your structure.- For butadiene only, the molecule must be in the s-cis configuration. To change the dihedral angle, click the cube to convert to 3D, and in the 3D structure editor, select all four carbon atoms and a slider should pop up, allowing you to change the angle.
- Click
Saveto exit the 3D editor, rename to butadiene, and repeat this process for ethylene, and cyclohexene (there are many ring structures in the bottom left of the 2D editor). - Set the
Level of Theoryto AIMNet2, selectOptimizeandFrequencies, and clickSubmit(this will submit all structures at once).
If any of your structures say that there is an "unexpected imaginary frequency," follow these steps:
- Click
Resubmit(below the 3D viewer) - Select
Displaced along a frequencyfrom the options that pop up, and select any negative frequency - Drag the slider all the way to the right before resubmitting as a calculation
- Set the
Level of Theoryto AIMNet2, selectOptimizeandFrequencies, and clickSubmit - Repeat for all calculations that return an "unexpected imaginary frequency"
Transition State
- Click the
View on Rowanbutton on the above reaction, and select "Step 0" (the peak) on the IRC. - Click the
Copy XYZbutton below the 3D viewer, and copy the coordinates. - Navigate to the Diels–Alder Lab folder you created earlier, click on
Calculation>⋮>Paste XYZ, paste the coordinates into the text box, and clickSubmit. - Select AIMNet2 in the
Level of Theorydropdown, selectOptimize (TS)andFrequencies, and clickSubmit.
Wait for the workflows to complete (≈2 minutes) and fill out the below table, where C1 and C2 correspond to the atoms in ethylene, and C3, C4, C5, and C6 are in butadiene.
For the energy, find the Electronic Energy (under Overview), and click on Hartree to switch units to kcal/mol. For bond lengths, select the two atoms you are looking for the distance between, and in the top left of the viewer, a box will appear with the distance between the atoms.
(Note, the energy for the reactants is the combined energy of the two molecules)
| Species | Energy (kcal/mol) | C1–C2 (Å) | C2–C3 (Å) | C3–C4 (Å) | C4–C5 (Å) | C5–C6 (Å) | C6–C1 (Å) |
|---|---|---|---|---|---|---|---|
| Reactants | – | – | |||||
| Transition State | |||||||
| Products |
What happens to the energy as we go from reactants to transition state to products? Is the reaction exothermic or endothermic? How do you know?
Draw an arrow in each column indicating whether the bond length increases or decreases as the reaction proceeds from reactants to products. How do the bond-lengths change over the course of the reaction, and what do you think is happening?
To find the activation energy of a reaction, you must subtract the energy of the reactants from the energy of the transition state. What is the activation energy of the basic Diels–Alder reaction?
To visualize the change in bond lengths, you can submit the transition state calculation as an IRC:
- Click
Resubmit(below the 3D viewer) >Current structure>Use this Selection, and selectResubmit as intrinsic reaction coordinate. - Make sure to AIMNet2 is selected in the
Level of Theorydropdown, and clickSubmit. - Wait for the job to complete (≈3 minutes), and select the bond of interest to observe how it changes over the course of the reaction.
Modifying the Rate of the Diels–Alder Reaction
- Create a new folder called Fluoro Group.
- Click on your butadiene, click
Resubmit(below the 3D viewer) >Current structure>Use this Selection, and selectResubmit as Calculation. - Use the 3D editor to add a fluorine to a middle carbon of the diene (replace a hydrogen with a fluorine from the
Periodic Tablebutton), and save when you are done (make sure not to disturb the rest of the structure). - Change the name of the workflow to "Diene - Fluoro", make sure the
Level of Theoryis set to AIMNet2 and thatOptimizeandFrequenciesare selected, and clickSubmit. - Repeat the process for the product and transition state ensuring that the floro group is in the same place (use
Optimize (TS)for the transition state). - Select the jobs and drag them into the Fluoro Group folder you created
- Wait for the jobs to complete (≈2 minutes) and fill out the below table.
(Note, the energy of the reactants is that of ethylene and the fluoro-substituted butadiene)
| Species | Energy (kcal/mol) | C1–C2 (Å) | C2–C3 (Å) | C3–C4 (Å) | C4–C5 (Å) | C5–C6 (Å) | C6–C1 (Å) |
|---|---|---|---|---|---|---|---|
| Reactants | – | – | |||||
| Transition State | |||||||
| Products |
What happens to the energy as we go from reactants to transition state to products? Is the reaction exothermic or endothermic? How do you know?
What is the activation energy, and how does this compare to the activation energy of the original Diels–Alder reaction? What does that imply about the relative reaction rate?
The fluoro group is an electron-withdrawing group. How do you predict the rate would change if a different electron-withdrawing group were used on the diene?
Based on your understanding, what would you expect if an electron-donating group were added to the diene? What if it were added to the dienophile?
Test your prediction: repeat the process above, but add a methyl group (CH3) to the dienophile. What did you observe? Did the reaction proceed faster or slower than the version without the methyl group?
| Species | Activation Energy (kcal/mol) |
|---|---|
| Base reaction | |
| Fluoro reaction | |
| Methyl reaction |
Do the bonding patterns in the products change when substituents are added? Do the added groups shift their bonding, or do they remain attached to the same atoms?
Other Factors Impacting the Diels–Alder Reaction
Does the location of the electron-donating and electron-withdrawing groups on the diene/dienophile affect the results? Try changing the location of one of the above r-groups and see what happens.
R-group: ______________
| Species | Location 1 Energy (kcal/mol) | Location 2 Energy (kcal/mol) |
|---|---|---|
| Reactants | ||
| Transition State | ||
| Products | ||
| Activation Energy |
Based on the bonding requirements of the Diels–Alder reaction, could ethyne serve as a dienophile? Why or why not? After making your predictions, test it out by following these steps, then write down your findings.
- Resubmit your transition state from the basic Diels–Alder reaction, and create a new calculation
- Use the
Remove Hbutton to remove a hydrogen from each carbon in the dienophile - Select AIMNet2 in the
Level of Theorydropdown, selectOptimize (TS)andFrequencies, and clickSubmit. - Once the transition state geometry has been optimized, click
Resubmit(below the 3D viewer) >Current structure>Use this Selection, and selectResubmit as intrinsic reaction coordinate. - Make sure to AIMNet2 is selected in the
Level of Theorydropdown, and clickSubmit. - Wait for the job to complete (≈3 minutes), and observe whether a six-membered ring forms.
Review
How does adding an electron-donating group to the dienophile affect the rate of reaction?
How does adding an electron-withdrawing group to the diene affect the rate of reaction?
What other ways could the rate of a Diels–Alder reaction be changed?
What other factors influence whether a Diels–Alder reaction will proceed?