Bond-Dissociation-Energy (BDE) Lab
by Sawyer VanZanten · Sept 9, 2025
Overview
This lab introduces the topic of bond-dissociation energy (BDE) (it's likely not what you think!), how to predict it, and how to calculate it using Rowan.
Background
A number you might have seen pop up in a chemistry course is bond-dissociation energy (BDE). BDE is often described as "the amount of energy needed to break (cleave) a bond." While this may seem like a simple concept, it's important to be careful here—what do we mean when we say "break a bond"?
Heterolytic Cleavage
Suppose we wanted to break the H–Cl single bond in hydrochloric acid. In a single bond, two electrons are shared between the two atoms in the bond. When we break the H–Cl bond, we separate the hydrogen atom from the chlorine atom such that no electrons can be shared between them. This means that each of the two electrons in the bond has to "pick a side"; each electron has to move to either the hydrogen atom or the chlorine atom.
Where will the electrons go when the H–Cl bond is broken? One possibility is that both of the electrons in the bond move to chlorine. A neutral chlorine atom has seven valence electrons, but after gaining both of the electrons from the bond, the chlorine atom would have eight electrons surrounding it—this would give it a negative charge. A neutral hydrogen atom has one valence electron, but after chlorine takes both electrons from the bond, the hydrogen atom wouldn't have any electrons surrounding it—this means hydrogen would have a positive charge. So, when the H–Cl bond breaks in this way, the H+ and Cl− ions are created.
Heterolytic cleavage of H–Cl produces the H+ and Cl- ions (note that the arrow here denotes the movement of two electrons)
When both of the electrons in a bond move to the same atom after the bond is broken, it is called heterolytic cleavage, or heterolysis. "Heterolysis" is Greek for "different splitting" (Gr. ἕτερος "different" and λύσις "loosing") and during heterolysis, something different happens to each of the two atoms in the bond. One atom (chlorine, in our case) gains both of the electrons from the bond, and the other atom (hydrogen) doesn't get any of the electrons from the bond. Poor hydrogen!
Heterolysis is a process you're likely familiar with. When HCl acts as an acid in an aqueous solution, the H–Cl bond breaks heterolytically, creating H+ and Cl− (the H+ created in this process usually bonds to water to create H3O+). While it's also theoretically possible for both of the electrons in the H–Cl bond to move to hydrogen when the bond breaks (creating H− and Cl+), this is very unlikely to happen, since chlorine is much more electronegative than hydrogen. Note that, when heterolysis happens in aqueous solution, the ions are stabilized by the polar solvent (water). The heterolysis of the H–Cl bond isn't nearly as favorable without this solvent stabilization.
Homolytic Cleavage
There's another way the H–Cl bond can be broken: one of the electrons in the bond can move to hydrogen, and the other one can move to chlorine. Breaking a bond such that one electron moves to each atom in the bond is called homolytic cleavage, or homolysis. "Homolysis" is Greek for "same splitting" (Gr. ὅμοιος "same" λύσις "loosing") and during homolysis, the same thing happens to each of the atoms in the bond: they both gain one of the electrons from the bond.
Splitting the H–Cl bond homolytically would not create ions, since chlorine would end up with seven valence electrons (the same as the number of valence electrons it has in its neutral state) and hydrogen would end up with one valence electron (the same as the number of valence electrons it has in its neutral state). Instead, two uncharged species would be created: H• and •Cl. Each of these species would have an unpaired electron (denoted by the dot in "H•" and "•Cl"). In chemistry, species with at least one unpaired electron have a special name: radicals. So we can say that breaking the H–Cl bond homolytically creates the H• radical and the •Cl radical. You might have seen homolysis before if you've taken organic chemistry: homolysis is the basis of free radical halogenation.
Homolytic cleavage of H–Cl produces the H• and Cl• radicals (note that the "fishhook" arrows here each denote the movement of only one electron)
Now that we've gone over heterolysis and homolysis, it's time to end the suspense: BDE measures the amount of energy it takes to break a bond homolytically. If we think about it, this makes sense: certain problems could arise if heterolysis were used instead. The heterolytic cleavage of a bond can, at least in theory, happen in two different ways—either of the atoms in the bond could take both of the electrons in the bond, potentially creating confusion about what is meant by "the heterolysis of the bond." Also, heterolysis creates ions, and ions can participate in all sorts of solvent effects that depend on the solvent the heterolysis is happening in. There's only one way a bond can homolytically cleave, and solvent effects are much less important for the uncharged radicals created by homolysis.
BDE measures the difference in energy between a starting molecule and the radicals produced after the molecule's bond is homolytically cleaved. If the radicals created by breaking the bond are low in energy, breaking the bond will be a relatively favorable process that doesn't require much energy, and the BDE will be small. Conversely, if breaking a bond homolytically creates two high-energy radicals, the BDE will be large.
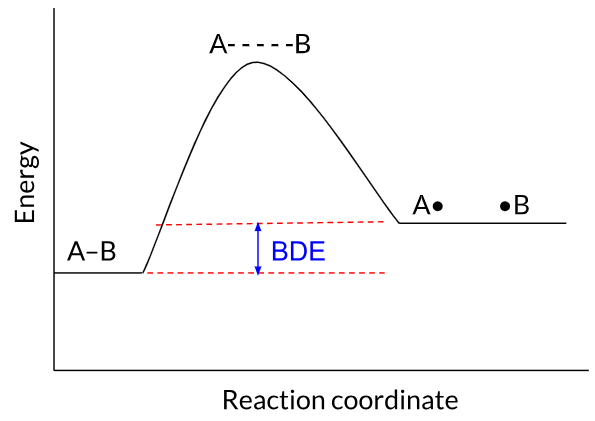
Because the radicals (A• and •B) produced when the A–B bond is homolytically cleaved are low in energy, the BDE is low.
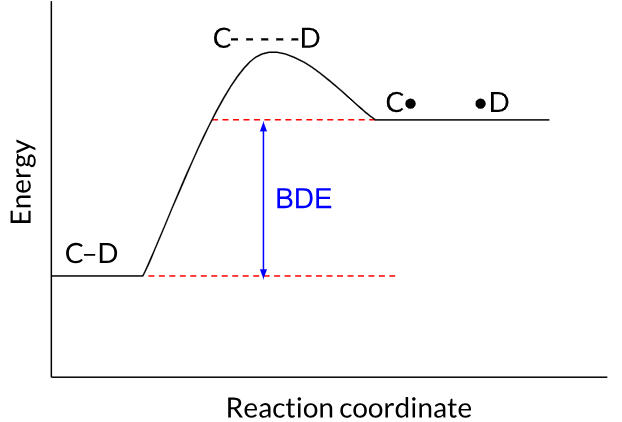
Because the radicals (C• and •D) produced when the C–D bond is homolytically cleaved are high in energy, the BDE is high.
How can you predict radical stability? The key thing to remember is that radicals are electron-deficient due to their unpaired electron. As you work through this lab, think about what factors would stabilize an electron-deficient species.
Lab
Task #1: Moving Across a Period
Let's investigate how BDE changes as the non-hydrogen atom involved in the bond moves to the right on the periodic table.
Do the following:
- Log in to Rowan (or create an account!), enter a project, and create a new folder called BDE Lab.
- Scroll down until you see the BDE Prediction workflow. Click on it, then click
Draw 2D. Draw the CH4 molecule by selectingsingle bondfrom the menu on the left side of the viewer, then clicking and dragging to create your bonds. - After drawing the molecule, click
Savein the top right. Click the cube icon to the right of the SMILES string to convert to 3D. - Click
Add Bond, and then click the carbon atom and any hydrogen atom in the CH4 structure. The BDE Prediction workflow calculates the amount of energy needed to homolytically cleave the bond between the two atoms you have selected. - Set the mode to Rapid NNP. Click
Submit BDEto submit the calculation. - Click the back arrow at the top left to go back to the workflow menu. Using the same steps as before, submit a BDE Prediction workflow for the N–H bond in NH3, the O–H bond in H2O, and the F–H bond in HF.
- When each calculation has finished, click it to view the result.
What is the trend in BDE as the central atom moves to the right on the periodic table (from carbon to nitrogen to oxygen to fluorine)? What might be the reason for this trend?
(Here's some information that might help: In a polar bond, the electrons are unevenly shared between the two atoms; the more electronegative atom has a greater share of the electrons in the bond. When a polar bond is broken, the previously unevenly-shared electrons become evenly distributed between the atoms, and thus the more electronegative atom has significantly less electron density than before.)
Despite being the most acidic, HF does not have the smallest BDE among the four species you ran calculations on. What is the reason for this?
Task #2: Moving Down a Group
Use Rowan to calculate the BDE of the bond in HF, HCl, HBr, and HI. What is the trend in BDEs as you move down the halogen group of the periodic table?
What might be the reason for this trend?
Task #3: Primary vs. Tertiary vs. Allylic vs. Benzylic
Use Rowan to calculate the BDE of:
- the primary C–H bond in methane
- the tertiary C–H bond in isobutane
- the allylic C–H bond in propene
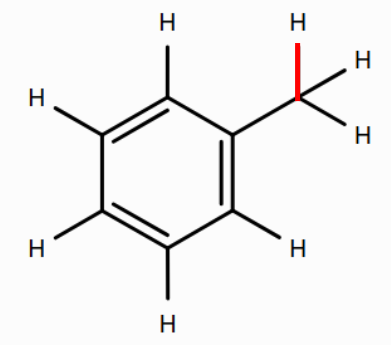
- the benzylic C–H bond in methylbenzene
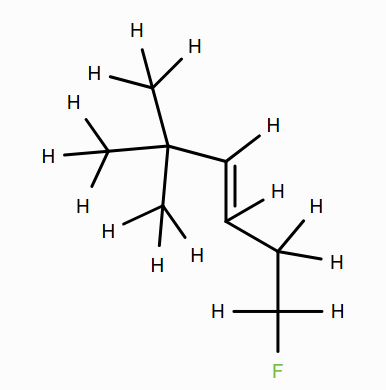
(See bonds colored red below.) Note that double-clicking a bond in the 2D editor allows you to turn it into a double bond.
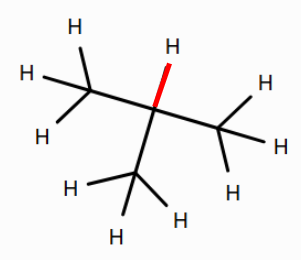
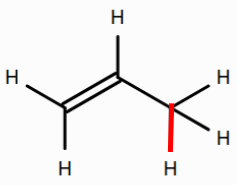
Does the primary C–H bond in methane or the tertiary C–H in isobutane have a greater BDE? What might be the reason for this?
Based on BDEs, is the methane radical or the propene radical more stable? What might be the reason for this? (Hint: What does the propene radical have that the methane radical lacks?)
Based on BDEs, is the propene radical or the methylbenzene radical more stable? Why might this be?
Task #4: Ethane vs. Ethene vs. Ethyne
Use Rowan to calculate the BDE of the bond between the carbon atom and the hydrogen atom in ethane, the bond between the carbon atom and the hydrogen atom in ethene, and the bond between the carbon atom and the hydrogen atom in ethyne.
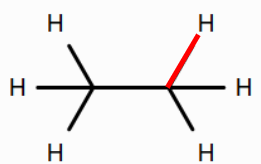
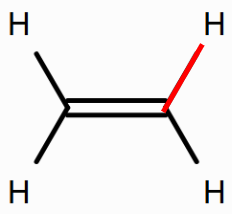
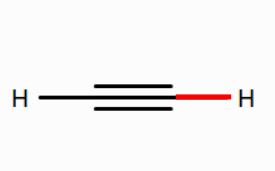
How does the BDE of the carbon-hydrogen bond change as the bond order of the carbon–carbon bond increases from one (ethane) to two (ethene) to three (ethyne)? What might be the reason for this? (Hint: As the bond order of the C-C bond increases, what property of the carbon atoms changes?)
Review
What is the difference between homolytic cleavage and heterolytic cleavage?
What is the definition of BDE?
Do you think the C–H bond in CH4 or the C–H bond in CHF3 would have a larger BDE? Why?
Based on what you've learned, which C–H bond in this molecule do you think would have the largest BDE? The smallest?